Pulmonary Emphysema Market Set to Expand by 2034 with Rising COPD Prevalence and Therapeutic Innovation | DelveInsight
The pulmonary emphysema market is poised for steady growth driven by rising COPD prevalence, an aging global population, and greater diagnostic awareness. Advances in inhaled therapies, targeted biologics, and minimally invasive interventions are expanding treatment options and market share. Additionally, the launch of therapies such as Tanimilast (CHF6001) (Chiesi Farmaceutici S.p.A.), SAR-447537 (Sanofi), Solrikitug (Uniquity Bio), PBF-680 (Palobiofarma), BMN-349 (BioMarin Pharmaceutical), and others will further accelerate the market growth.
New York, USA, Nov. 03, 2025 (GLOBE NEWSWIRE) -- Pulmonary Emphysema Market Set to Expand by 2034 with Rising COPD Prevalence and Therapeutic Innovation | DelveInsight
The pulmonary emphysema market is poised for steady growth driven by rising COPD prevalence, an aging global population, and greater diagnostic awareness. Advances in inhaled therapies, targeted biologics, and minimally invasive interventions are expanding treatment options and market share. Additionally, the launch of therapies such as Tanimilast (CHF6001) (Chiesi Farmaceutici S.p.A.), SAR-447537 (Sanofi), Solrikitug (Uniquity Bio), PBF-680 (Palobiofarma), BMN-349 (BioMarin Pharmaceutical), and others will further accelerate the market growth.
DelveInsight’s Pulmonary Emphysema Market Insights report includes a comprehensive understanding of current treatment practices, emerging pulmonary emphysema drugs, market share of individual therapies, and current and forecasted pulmonary emphysema market size from 2020 to 2034, segmented into leading markets (the US, EU4, UK, and Japan).
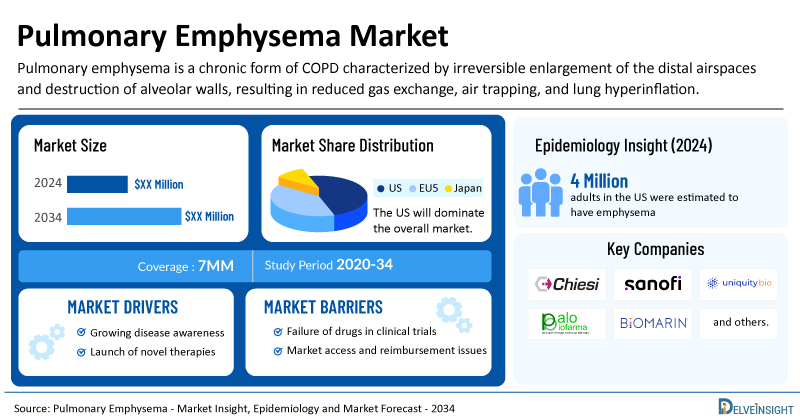
Pulmonary Emphysema Market Summary
- The total pulmonary emphysema treatment market size is expected to grow positively by 2034 in the leading markets.
- The United States accounts for the largest market size of pulmonary emphysema, in comparison to EU4 (Germany, Italy, France, and Spain), the UK, and Japan.
- NUCALA became the second biologic approved for COPD with eosinophilic inflammation, following closely after DUPIXENT’s breakthrough in 2024.
- TRELEGY ELLIPTA and BREZTRI AEROSPHERE continue to drive triple therapy adoption, leveraging once-daily dosing, device convenience, and broad market access.
- In 2024, approximately 4 million adults in the US were estimated to have emphysema.
- Key pulmonary emphysema companies, including Chiesi Farmaceutici S.p.A., Sanofi, Uniquity Bio, Palobiofarma, BioMarin Pharmaceutical, and others, are actively working on innovative pulmonary emphysema drugs.
- Some of the key pulmonary emphysema therapies in clinical trials include Tanimilast (CHF6001), SAR-447537, Solrikitug, PBF-680, BMN-349, and others. These novel pulmonary emphysema therapies are anticipated to enter the pulmonary emphysema market in the forecast period and are expected to change the market.
- OHTUVAYRE by Verona Pharma is anticipated to capture the maximum market share among emerging therapies by 2034.
Discover which pulmonary emphysema medications are expected to grab the market share @ Pulmonary Emphysema Market Report
Key Factors Driving the Growth of the Pulmonary Emphysema Market
Rising Prevalence of Emphysema and COPD
The increasing prevalence of COPD, including emphysema, is a primary driver. Factors such as smoking, air pollution, and genetic predispositions contribute to this rise. In 2024, DelveInsight estimated that the 7MM had around 31 million diagnosed cases of COPD, including approximately 8.5 million cases of emphysema, with prevalence projected to grow throughout the forecast period (2025–2034).
Advancements in Pulmonary Emphysema Treatment Options
Innovations in pharmacological treatments, including bronchodilators, inhaled steroids, and biologics, have improved patient outcomes. Notably, drugs like DUPIXENT and OHTUVAYRE have shown efficacy in treating COPD and are expanding the therapeutic landscape.
Launch of Emerging Pulmonary Emphysema Drugs
Some of the emphysema drugs in clinical trials include Tanimilast (CHF6001) (Chiesi Farmaceutici S.p.A.), SAR-447537 (Sanofi), Solrikitug (Uniquity Bio), PBF-680 (Palobiofarma), BMN-349 (BioMarin Pharmaceutical), and others. The anticipated launch of these drugs is expected to drive the pulmonary emphysema market forward in the coming years.
Pulmonary Emphysema Market Analysis
Pulmonary emphysema, a key feature of COPD, involves gradual destruction of lung tissue, persistent airflow obstruction, and significant morbidity. Current treatment options primarily aim to alleviate symptoms and include bronchodilators (LABAs, LAMAs), inhaled corticosteroids (ICS), and fixed-dose combinations such as beclometasone/formoterol/glycopyrronium bromide (TRIMBOW), along with mucolytics to enhance airflow and reduce exacerbations. However, these therapies do not stop disease progression.
Targeted interventions, including biologics and small molecules such as dupilumab (DUPIXENT), mepolizumab (NUCALA), and ensifentrine (OHTUVAYRE), are used for patients with eosinophilic inflammation or those who experience frequent flare-ups. For cases linked to alpha-1 antitrypsin deficiency (AATD), plasma-derived AAT augmentation therapy remains the standard of care to slow lung damage. The emerging drug pipeline is focused on disease-modifying strategies that aim to restore functional AAT, preserve alveolar integrity, and potentially alter disease trajectory. Notable investigational agents include Solrikitug (Uniquity Bio), PBF-680 (Palobiofarma), Tanimilast/CHF6001 (Chiesi Farmaceutici S.p.A.), long-acting recombinant AAT fusion proteins (SAR-447537), and small-molecule chaperones (BMN-349).
Learn more about the pulmonary emphysema treatment options @ Pulmonary Emphysema Treatment Market
Pulmonary Emphysema Competitive Landscape
The pipeline for treating pulmonary emphysema includes Tanimilast (CHF6001) (Chiesi Farmaceutici S.p.A.), SAR-447537 (Sanofi), Solrikitug (Uniquity Bio), PBF-680 (Palobiofarma), BMN-349 (BioMarin Pharmaceutical), and others currently in development.
Sanofi’s SAR-447537 (previously known as INBRX-101) is an experimental recombinant fusion protein being developed by Sanofi to treat alpha-1 antitrypsin deficiency (AATD), a rare inherited disorder that can lead to pulmonary emphysema. Engineered as a long-acting AAT-Fc fusion protein, it is designed to sustain protective AAT levels in both the bloodstream and lung tissue with dosing every three to four weeks—offering a less frequent alternative to current plasma-derived therapies that require weekly administration. By preserving alveolar integrity and limiting protease-mediated tissue damage, SAR-447537 has the potential to slow the progression of emphysema. The therapy holds orphan drug designation and is currently being evaluated in the Phase II ElevAATe trial, as well as a long-term open-label extension study. Preliminary findings demonstrate durable AAT activity and effective lung delivery, indicating potential benefits in treatment convenience, adherence, and long-term outcomes for individuals with AATD-associated emphysema.
Chiesi Farmaceutici S.p.A.’s CHF6001, also known as Tanimilast, is an anti-inflammatory agent under development for the treatment of respiratory disorders, including COPD and asthma. It acts as a phosphodiesterase-4 (PDE4) inhibitor, blocking the breakdown of cyclic adenosine monophosphate (cAMP) within inflammatory cells. This inhibition reduces inflammation and modulates immune activity, thereby helping to alleviate symptoms and improve lung function in patients with chronic inflammatory airway diseases.
The anticipated launch of these emerging pulmonary emphysema therapies are poised to transform the Pulmonary Emphysema market landscape in the coming years. As these cutting-edge pulmonary emphysema therapies continue to mature and gain regulatory approval, they are expected to reshape the pulmonary emphysema market landscape, offering new standards of care and unlocking opportunities for medical innovation and economic growth.
To know more about new treatment for pulmonary emphysema, visit @ Pulmonary Emphysema Medication
What is Pulmonary Emphysema?
Pulmonary emphysema is a chronic form of COPD characterized by irreversible enlargement of the distal airspaces and destruction of alveolar walls, resulting in reduced gas exchange, air trapping, and lung hyperinflation. The primary cause is cigarette smoking, though α1-antitrypsin deficiency and environmental pollutants also contribute. Patients typically present with gradually worsening shortness of breath, little to no cough, and the classic “pink puffer” appearance.
Pulmonary Emphysema Epidemiology Segmentation
The pulmonary emphysema epidemiology section provides insights into the historical and current pulmonary emphysema patient pool and forecasted trends for the leading markets. The highest prevalence of emphysema is observed among individuals aged 55 to 65 years and older, indicating an increased risk of disease with advancing age.
The pulmonary emphysema market report proffers epidemiological analysis for the study period 2020–2034 in the leading markets segmented into:
- Total Diagnosed Prevalent Cases of COPD
- Total Diagnosed Prevalent Cases of Pulmonary Emphysema
- Age-specific Diagnosed Prevalent Cases of Pulmonary Emphysema
- Total Treatable Cases of Pulmonary Emphysema
Download the report to understand pulmonary emphysema management @ Pulmonary Emphysema Treatment Options
| Pulmonary Emphysema Market Report Metrics | Details |
| Study Period | 2020–2034 |
| Pulmonary Emphysema Market Report Coverage | 7MM [The United States, the EU-4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan] |
| Pulmonary Emphysema Epidemiology Segmentation | Total Diagnosed Prevalent Cases of COPD, Total Diagnosed Prevalent Cases of Pulmonary Emphysema, Age-specific Diagnosed Prevalent Cases of Pulmonary Emphysema, and Total Treatable Cases of Pulmonary Emphysema |
| Key Pulmonary Emphysema Companies | Chiesi Farmaceutici S.p.A., Sanofi, Uniquity Bio, Palobiofarma, BioMarin Pharmaceutical, Verona Pharma, Merck, Regeneron Pharmaceuticals, and others |
| Key Pulmonary Emphysema Therapies | Tanimilast (CHF6001), SAR-447537, Solrikitug, PBF-680, BMN-349, OHTUVAYRE, DUPIXENT, NUCALA, and others |
Scope of the Pulmonary Emphysema Market Report
- Pulmonary Emphysema Therapeutic Assessment: Pulmonary Emphysema current marketed and emerging therapies
- Pulmonary Emphysema Market Dynamics: Conjoint Analysis of Emerging Pulmonary Emphysema Drugs
- Competitive Intelligence Analysis: SWOT analysis and Market entry strategies
- Pulmonary Emphysema Market Unmet Needs, KOL’s views, Analyst’s views, Pulmonary Emphysema Market Access and Reimbursement
Discover more about pulmonary emphysema drugs in development @ Pulmonary Emphysema Clinical Trials
Table of Contents
| 1 | Pulmonary Emphysema Market Key Insights |
| 2 | Pulmonary Emphysema Market Report Introduction |
| 3 | Epidemiology and Market Forecast Methodology |
| 4 | Pulmonary Emphysema: Market Overview at a Glance |
| 4.1 | Total Market Share (%) Distribution of Pulmonary Emphysema by Therapies in 2024 |
| 4.2 | Total Market Share (%) Distribution of Pulmonary Emphysema by Therapies in 2034 |
| 5 | Executive Summary |
| 6 | Key Events |
| 7 | Disease Background and Overview: Pulmonary Emphysema |
| 7.1 | Introduction |
| 7.2 | Pulmonary Emphysema Causes |
| 7.3 | Pulmonary Emphysema Pathophysiology |
| 7.4 | Pulmonary Emphysema Symptoms |
| 7.5 | Pulmonary Emphysema Risk Factor |
| 7.6 | Pulmonary Emphysema Diagnosis |
| 8 | Pulmonary Emphysema Treatment and Management |
| 8.1 | Treatment Guidelines |
| 9 | Epidemiology and Patient Population |
| 9.1 | Key Findings |
| 9.2 | Assumptions and Rationale |
| 9.3 | Total Diagnosed Prevalent cases of Pulmonary Emphysema:7MM |
| 9.4 | The United States |
| 9.4.1 | Total Diagnosed Prevalent Cases of COPD in the United States |
| 9.4.2 | Total Diagnosed Prevalent Cases of Pulmonary Emphysema in the United States |
| 9.4.3 | Age-specific Diagnosed Prevalent Cases of Pulmonary Emphysema in the United States |
| 9.4.4 | Total Treatable Cases of Pulmonary Emphysema in the United States |
| 9.5 | EU4 and the UK |
| 9.6 | Japan |
| 10 | Patient Journey of Pulmonary Emphysema |
| 11 | Marketed Pulmonary Emphysema Drugs |
| 11.1 | Key Competitors |
| 11.2 | Dupilumab (DUPIXENT): Regeneron Pharmaceuticals/Sanofi |
| 11.2.1 | Product Description |
| 11.2.2 | Regulatory Milestones |
| 11.2.3 | Other Developmental Activities |
| 11.2.4 | Clinical Developmental Activities |
| 11.2.5 | Safety and Efficacy |
| 11.2.6 | Analyst Views |
| 11.3 | Ensifentrine (OHTUVAYRE): Verona Pharma |
| List to be continued in the report… | |
| 12 | Emerging Pulmonary Emphysema Therapies |
| 12.1 | Key Cross Competition |
| 12.2 | SAR447537: Sanofi |
| 12.2.1 | Product Description |
| 12.2.2 | Other Development Activities |
| 12.2.3 | Clinical Development Activities |
| 12.2.4 | Safety and Efficacy |
| 12.2.5 | Analyst View |
| 12.3 | BMN-349: BioMarin Pharmaceutical |
| List to be continued in the report… | |
| 13 | Pulmonary Emphysema: Market Size |
| 13.1 | Key Findings |
| 13.2 | Pulmonary Emphysema Market Outlook |
| 13.3 | Conjoint Analysis |
| 13.4 | Key Pulmonary Emphysema Market Forecast Assumptions |
| 13.6 | United States Pulmonary Emphysema Market Size |
| 13.6.1 | Total Market Size of Pulmonary Emphysema by Indication in the United States |
| 13.6.2 | Market Size of Pulmonary Emphysema by Therapies in the United States |
| 13.7 | EU4 and the UK Pulmonary Emphysema Market Size |
| 13.8 | Japan Pulmonary Emphysema Market Size |
| 14 | Unmet Needs of Pulmonary Emphysema |
| 15 | SWOT Analysis of Pulmonary Emphysema |
| 16 | KOL Views of Pulmonary Emphysema |
| 17 | Market Access and Reimbursement |
| 17.1 | United States |
| 17.2 | EU4 and the UK |
| 17.3 | Japan |
| 18 | Bibliography |
| 19 | Pulmonary Emphysema Market Report Methodology |
Related Reports
Emphysema Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key emphysema companies, including Mereo BioPharma, Beam Therapeutics, Regeneron Pharmaceuticals, Sanofi, GSK, AstraZeneca, Verona Pharma, among others.
Emphysema Clinical Trial Analysis
Emphysema Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key emphysema companies, including Wave Life Sciences, Vertex Pharmaceuticals, Takeda, Beam Therapeutics, Kamada, Gain Therapeutics, Arrowhead Pharmaceuticals, among others.
Chronic Obstructive Pulmonary Disease Market
Chronic Obstructive Pulmonary Disease Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key COPD companies including Genentech, Inc., GlaxoSmithKline, Verona Pharma plc, Regeneron Pharmaceuticals, Sanofi, MedImmune LLC, EpiEndo Pharmaceuticals, Tetherex Pharmaceuticals, AstraZeneca, Chiesi Farmaceutici S.p.A., Synairgen Research Ltd., Mereo Biopharma, Organicell Regenerative Medicine, Pulmotect, Inc., Inmunotek S.L., PULMATRiX, GLENMARK PHARMACEUTICALS LTD, Dimerix Limited, ProterixBio, among others.
Chronic Obstructive Pulmonary Disease Clinical Trial Analysis
Chronic Obstructive Pulmonary Disease Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key COPD companies, including Sanofi, Chiesi Farmaceutici S.p.A., United Therapeutics Corporation, Verona Pharma plc, Immunotek, Yungjin Pharm. Co., Ltd., Pulmotect, Inc., Tetherex Pharmaceutical, CSL Behring, AstraZeneca, Novartis, Genentech, Vertex Pharmaceuticals, EmeraMed, Afimmune, Mereo BioPharma, Synairgen, Adamis Pharmaceuticals, Quercegen Pharmaceuticals LLC, Regend Therapeutics, Meridigen Biotech Co., Ltd., Pulmatrix, Eisai, GlaxoSmithKline, EpiEndo Pharmaceuticals, 3SBio, OmniSpirant, Foresee Pharmaceuticals, Amgen, Organicell Regenerative Medicine, Arrowhead Pharmaceuticals, ProterixBio, RS BioTherapeutics, MitoRx, C4X Discovery, Respiratorius, ARK biosciences, Incannex, GNI Pharma, Celon pharma, Alveolus Bio, Kinaset therapeutics, Landos Biopharma, Parion Sciences, KeyMed Biosciences, Bioneer corporation, AlgiPharma, Palobiofarma, Dimerix Bioscience, Glenmark Pharmaceuticals, among others.
Chronic Obstructive Pulmonary Disease Treatment Devices Market
Chronic Obstructive Pulmonary Disease Treatment Devices Market Insights, Competitive Landscape, and Market Forecast – 2032 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key COPD treatment devices companies, including Koninklijke Philips N.V., Omron Healthcare, Inc., Aerogen, GF Health Products, Inc., BMC Medical Co., Ltd., Medtronic, Trudell Medical International, Novartis AG, AstraZeneca, Lupin, Zydus Cadila, Teva Pharmaceutical Industries Ltd, Terumo Corporation, Cipla Inc., Microlife Corporation, Honsun, Promed Technology Co. Limited, HELTMAN Medikal A.S., Pneuma Respiratory, Lepu Medical Technology (Beijing) Co., Ltd., among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Pharmaceutical Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Connect with us on LinkedIn|Facebook|Twitter

Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.